How to make bubbles "everlasting"?
Published on 27 August 2022
Kids love bubbles, and so do adults. Apart from the shifting appearance, bubbles are full of science. Let's take a closer look!
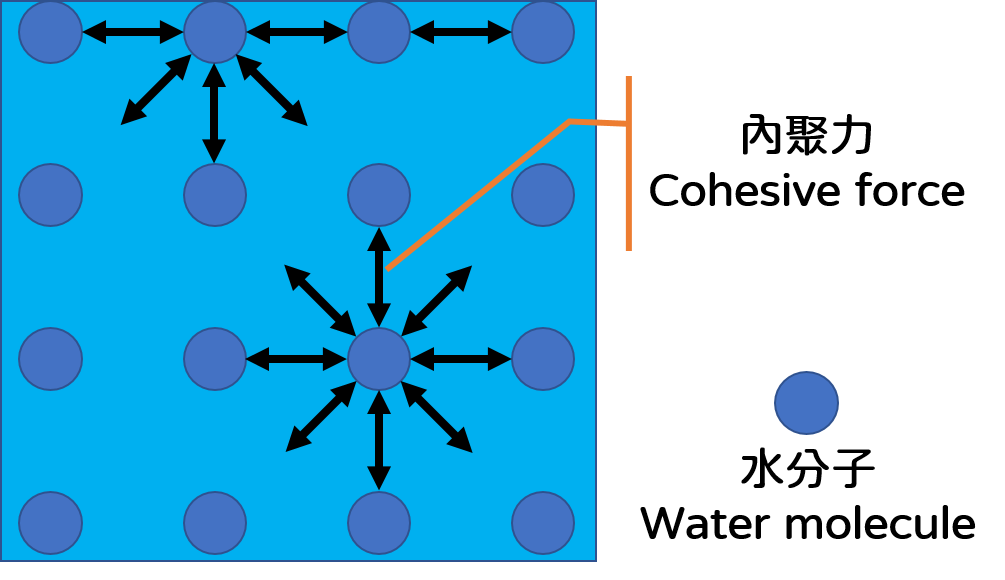
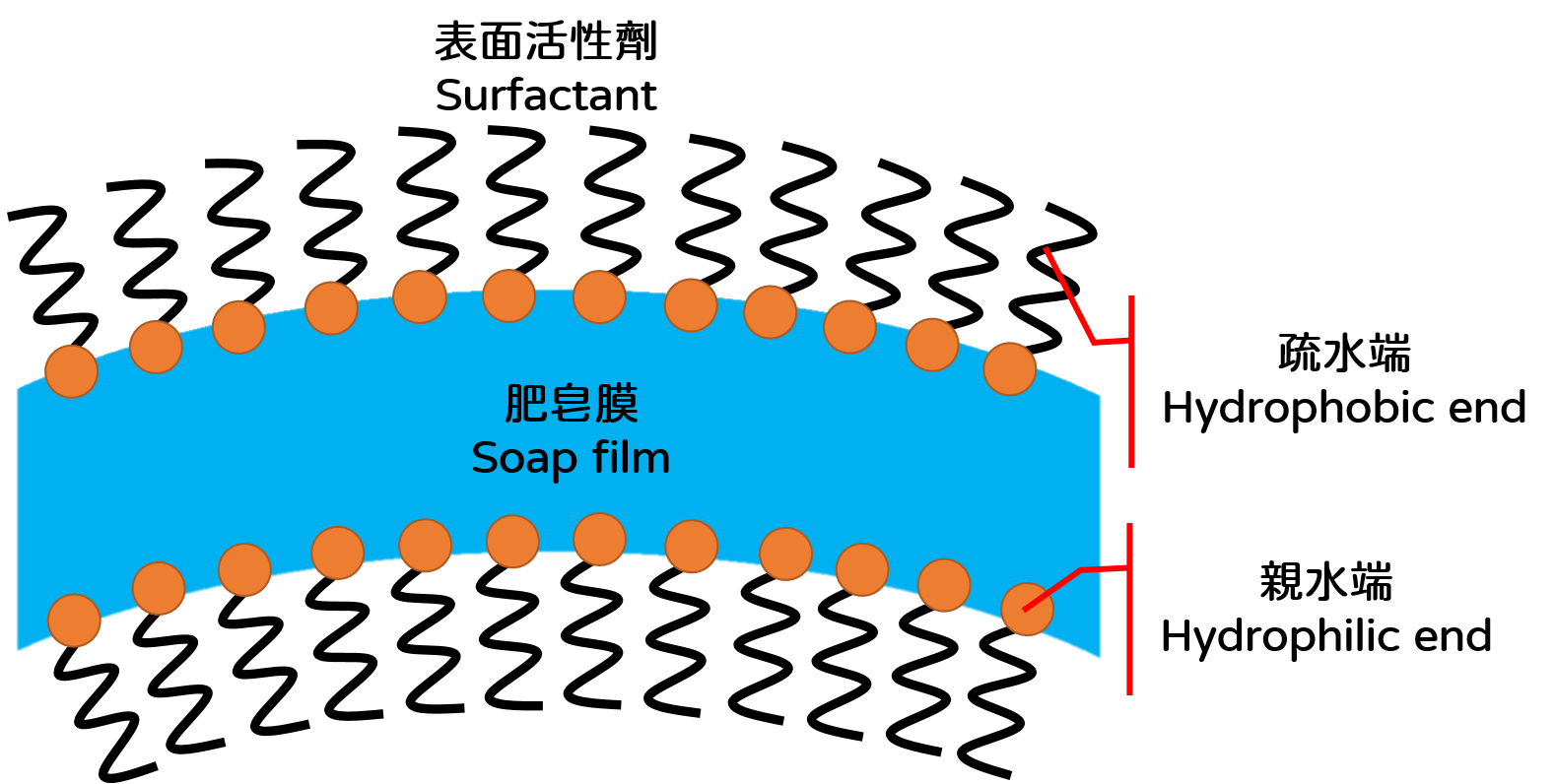
Bubbles formed by blowing air into plain water through a straw do not last long. It is because of gravity and the surface tension of water. Gravity thins the top of the bubble by pulling the water downward and the surface tension of the water speeds up the rupture of bubble from the weakest part like a punctured balloon. The surface tension of water comes from the cohesive forces between water molecules. The cohesive forces make the water molecules stay as close as possible to each other and the air-water interface tend to reach the smallest area, like a stretched rubber sheet with tension (Figure 1). Bubble solution can form soap bubbles because it has "surfactant" that reduces the surface tension of water. The molecular structure of a surfactant is special, with a "hydrophilic end" that likes water and a "hydrophobic end" that hates water. When we blow a soap bubble, the surfactants gather at the air-water interface to keep their hydrophobic end as far away as possible from the water. As a result, the soap film will have a layer of surfactant on the inner and outer surfaces, trapping the water in between (Figure 2).
Everyone who has played with soap bubbles will find that sometimes they burst very soon. It is because besides gravity, the evaporation of water in the bubble solution is another cause for the rupture of bubbles is. This year, a scientific team from the University of Lille in France announced that by adding glycerol and microparticles of plastic into the solution they created bubbles that lasted for 465 days. Unfortunately, these bubbles are too heavy to float in the air.
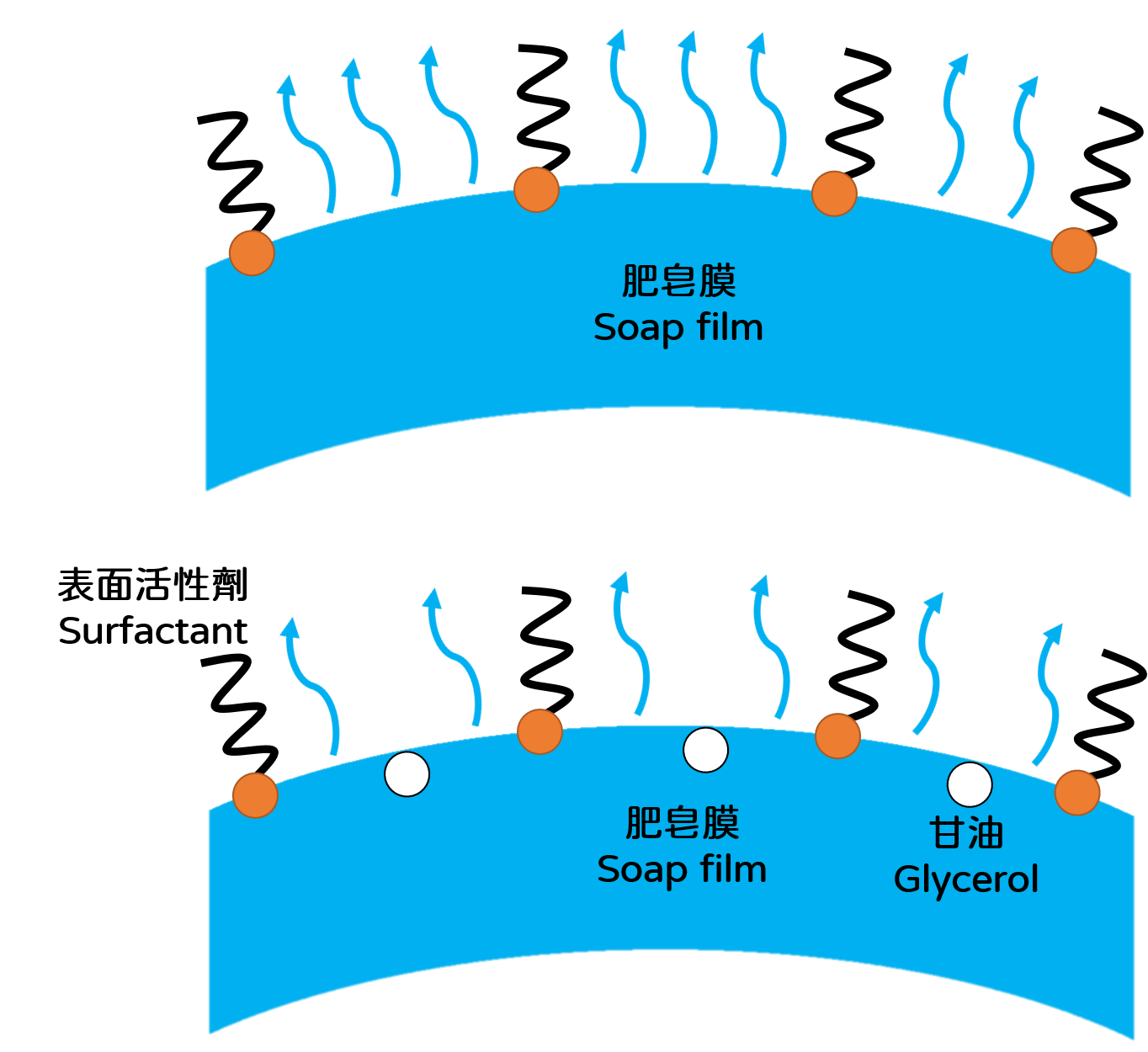
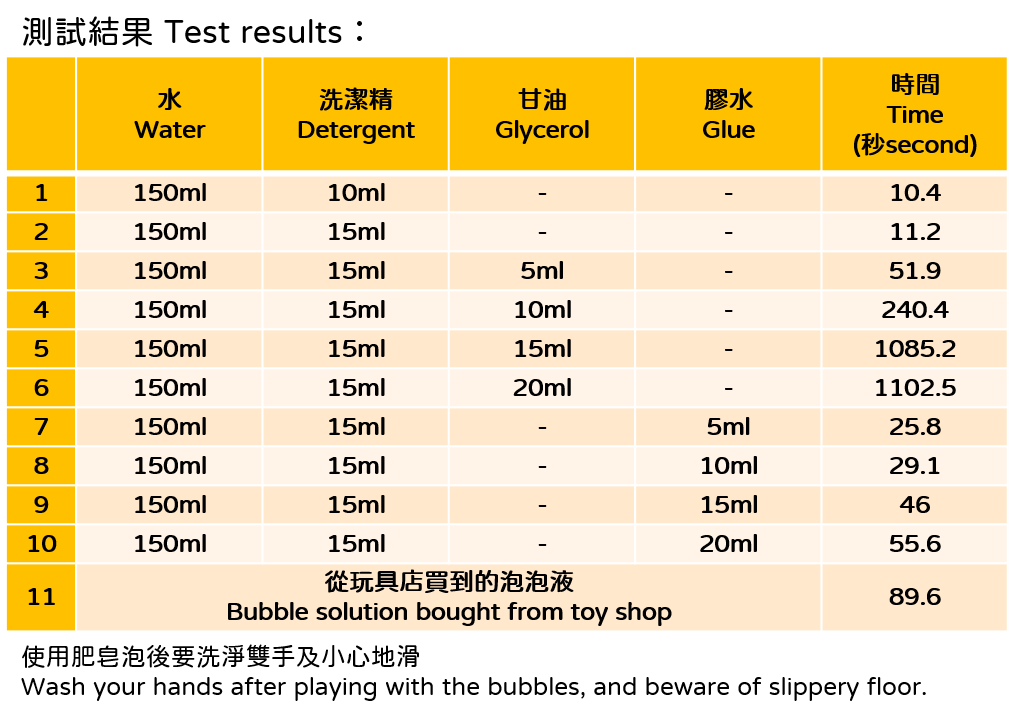
The soap bubbles for fun may not need to last that long, but still it is interesting to explore how to prepare a long-lasting bubble solution at home. Formulas found on the internet almost always contain detergent, glycerol and glue. Detergent provides surfactants, and glue contains polyvinyl alcohol (PVA), a polymer that increases the viscoelasticity, hence the strength, of the soap film. It especially important in making blowing giant bubbles. In addition, glycerol we mentioned above forms hydrogen bonds with water molecules and slow down the evaporation of water (Figure 3). But despite its effectiveness, glycerol cannot be added too much because it is denser than water, too much glycerol will make the soap bubbles heavier and easier to fall. After knowing the properties of these ingredients and by referring to the results of our experiment, you can also try making your own formula to see if you can create light and long-lasting bubbles!
Besides the bubble solution formula, environmental factors also affect how soap bubbles behave. On days with high humidity, the evaporation rate of the bubble solution will be slower and the bubbles will last longer. For another example, on a sunny day, the air close to the ground will be heated up and expanded to form an upward current. Soap bubbles can then ride on the current and fly higher. What else can you think of to make bubbles float in air?
1) Water 150ml
2) Detergent 15ml
3) Glycerol 20ml
4) Glue 20ml